|
|
|
You can get e-magazine links on WhatsApp. Click here
|
|
|
|
|
|
US FDA registration for food facilities in India: A gateway to global markets
|
|
Thursday, 22 August, 2024, 13 : 00 PM [IST]
|
|
Surabhi Soral
|
The Food Processing (FP), sector has become a key component of the Indian economy, significantly contributing to GDP, employment, and exports. According to a Ministry of Food Processing Industries report, the FP sector has grown at an Average Annual Growth Rate (AAGR), of approximately 7.26% in the last few years. The sector's Gross Value Added (GVA), has risen from Rs 1.30 lakh crore in 2013-14 to Rs 2.08 lakh crore in 2021-22.
The US and India are among the top food-producing nations globally, offering significant opportunities for collaboration and strengthening the sector's foundation. The US is India's largest market for F&B exports. India ranks 7th among the US's F&B import sources, with imports valued at US$ 6.4 billion in 2022, reflecting a 4-year CAGR of 7% and a 2.95% market share. These numbers are expected to grow exponentially in the coming years.
The US Food and Drug Administration (FDA), is a federal regulatory body within the Department of Health and Human Services (DHHS). As a scientific authority, the FDA is charged with safeguarding the safety of food (including additives), food contact materials, medical devices, radiological products, and pharmaceuticals that are manufactured or imported into United States. Before these products can be sold in US market, they must be registered or certified by the FDA.
The US FDA registration is mandatory for all food facilities that manufacture, process, pack, or store food for consumption in the United States. This regulation applies equally to domestic and foreign food facilities, including those in India. Currently, 5414 food business operators in India are registered with the US FDA. The facility's registration with the FDA must be renewed every two years between October 1 and December 31 of each even-numbered year. It's also important to allow FDA facility inspections as mandated by the FD&C Act.
The Registration Process
The USFDA registration process for food facilities is relatively straightforward but requires attention to detail. Here are the key steps involved: 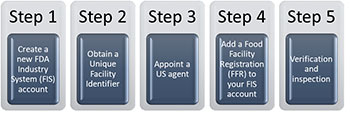 - Submission of Information/ FIS Account: Food facilities must submit detailed information about their operations, including the types of food products they handle, the processes they use, and the locations of their facilities. This information must be updated biennially.
- Unique facility identifier: The Data Universal Numbering System, also known as DUNS number is a nine-digit unique identifier for businesses across the world. the DUNS number is the preferred UFI recognized as acceptable to the FDA for food facility registration.
- Appointment of a U.S. Agent: Foreign facilities, including those in India, are required to appoint a U.S. agent who acts as a communication link between the USFDA and the facility. The agent must be physically located in the United States and is responsible for receiving communications from the FDA on behalf of the facility.
- Add a Food Facility Registration (FFR) to the FIS account: FBOs are required to log in to the FDA Industry Systems (FIS) website to create their FDA account.
- Verification and Inspection: The USFDA reserves the right to inspect registered facilities to verify compliance with U.S. regulations. Non-compliance can lead to enforcement actions, including the suspension of registration and the detention of products.
The impact of USFDA registration on the Indian food processing industry has been significant. According to data from the Agricultural and Processed Food Products Export Development Authority (APEDA), the U.S. is one of the top export destinations for Indian food products, accounting for over 10% of the country’s total food exports in 2022-23. USFDA registration has facilitated this growth by enabling Indian food manufacturers to meet the safety and quality expectations of U.S. consumers. Moreover, USFDA registration has encouraged Indian food facilities to upgrade their operations and adopt international best practices in food safety and hygiene. This has not only improved the quality of products exported to the U.S. but has also had a positive spillover effect on the domestic market, where consumers are increasingly demanding high-quality and safe food products. By ensuring compliance with stringent U.S. food safety regulations, Indian food manufacturers can enhance their credibility, avoid regulatory pitfalls, and capitalize on the growing demand for high-quality food products in the United States. As the Indian food processing industry continues to grow, USFDA registration will remain a vital tool for unlocking global markets and achieving sustained export success. (The author is head of regulatory and compliance services
at Food Safety Works)
|
|
|
|
|
|
|
|
|
|