|
|
|
You can get e-magazine links on WhatsApp. Click here
|
|
|
|
|
|
Novel ingredients - Common principles guide evaluation
|
|
Monday, 24 June, 2024, 16 : 00 PM [IST]
|
|
Ganesh Gaikwad, Dr R B Kshirsagar, Dr R P Mane
|
Food ingredients are substances used in the preparation or production of food to enhance flavour, texture, appearance or nutritional value. They can be natural or synthetic and are typically listed on food labels. Examples of some common food ingredients: Salt, Sugar, Flour, Oil, Spices, Herbs, Eggs, Milk, Yeast and Baking Powder.
Understanding food ingredients is essential for making informed dietary choices and for ensuring food safety and quality. Labels on food products typically list ingredients in descending order of predominance by weight, allowing consumers to know what they're consuming and make choices based on their dietary needs and preferences.
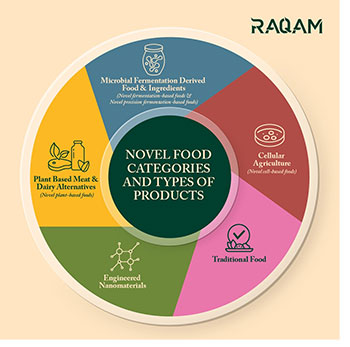
Novel food ingredients are those that have not been traditionally used in food preparation or consumption and are relatively new to the market. These ingredients often undergo thorough safety assessments before being approved for use in food products. They can come from various sources, including plants, animals, microorganisms, or chemical synthesis.
Novel food ingredients refer to substances that have not been traditionally used for human consumption to a significant degree within the European Union (EU) before May 15, 1997. These can include newly developed food products, foods derived from new processes, or foods produced using new technologies. The term also encompasses foods derived from new sources, such as those derived from genetically modified organisms (GMOs) or foods with modified molecular structures.
New Foods: Novel food ingredients include newly developed food products that were not commonly consumed before the specified date in 1997. These could be innovative food items created through advanced food processing techniques, such as novel flavours, colours or textures.
Novel Processes: Foods produced using new processes or technologies that significantly alter their composition, nutritional value, or safety profile may fall under the category of novel food ingredients. For instance, foods processed using novel preservation methods or extraction techniques may be considered novel.
New Sources: Ingredients derived from new sources also qualify as novel food ingredients. This includes foods derived from plants, animals, microorganisms, or other biological entities that were not traditionally consumed as food. For example, algae-based products or insect-derived ingredients are considered novel sources.
Genetically Modified Organisms (GMOs): Foods derived from genetically modified organisms, where the genetic material has been altered in a way that does not occur naturally, are typically classified as novel food ingredients. GMOs can include crops engineered for enhanced nutritional content, improved shelf life, or resistance to pests or diseases.
Modified Molecular Structures: Food ingredients that have undergone modifications at a molecular level, resulting in altered properties, functionalities, or health benefits, may also be considered novel. This includes novel food additives, enzymes, or other substances used to enhance food quality or safety.
A regulatory framework is a system of rules, regulations, laws, and guidelines established by governments or regulatory bodies to oversee and control various aspects of society, particularly in sectors such as finance, healthcare, environment, telecommunications, and more. It sets the standards and procedures that individuals, businesses, and organisations must follow to ensure compliance with legal requirements and maintain order in the respective industry or sector.
Regulatory authorities, such as the European Food Safety Authority (EFSA) in the EU, assess novel food ingredients for their safety, nutritional adequacy, and potential impact on consumer health. Before any novel food ingredient can be marketed and sold within the EU, it must undergo a thorough safety evaluation and receive authorisation from regulatory agencies. This process involves rigorous scientific assessment, including toxicological studies, allergenicity assessments, and compositional analyses, to ensure that the novel food ingredient is safe for human consumption.
Overall, the classification of a food ingredient as novel depends on factors such as its history of consumption, the nature of its production process, and its potential impact on human health and the environment. Regulatory frameworks aim to strike a balance between promoting innovation in the food industry and safeguarding public health and consumer interests.
Quinoa, a seed native to South America, gained popularity in recent years due to its nutritional benefits and versatility in cooking. While it has been consumed for centuries by indigenous peoples, it only became widely known in Western countries in the late 20th century. As a novel food ingredient, quinoa has been incorporated into various products such as cereals, snacks, and gluten-free flour blends. Its high protein content, unique texture and nutty flavour make it a popular choice for health-conscious consumers seeking alternative grains.
The regulatory framework for novel food ingredients varies across different countries and regions, but there are common principles that guide the evaluation and approval process. Here, I'll provide an overview of the regulatory framework typically followed in various jurisdictions, such as the European Union and United States.
In the EU, novel food regulation is governed by Regulation 2015/2283. This regulation defines novel foods as those that have not been consumed to a significant degree within the EU before May 1997. Novel foods can include newly developed foods, foods produced using new technologies and production processes, as well as foods traditionally consumed outside of the EU.
Key steps in the EU's regulatory framework for novel food ingredients include:
Novel Food Application: The applicant submits a novel food application to the European Food Safety Authority (EFSA), providing comprehensive scientific data on the safety and nutritional profile of the novel food.
Scientific Evaluation: EFSA conducts a thorough scientific evaluation of the novel food application, assessing its safety for human consumption, nutritional value, and any potential allergenicity or toxicity concerns.
Risk Assessment: EFSA evaluates the potential risks associated with the novel food ingredient, considering factors such as its composition, intended use, and potential exposure levels.
Public Consultation: EFSA may seek public consultation on the novel food application, allowing stakeholders and interested parties to provide feedback and additional information.
Approval or Rejection: Based on EFSA's scientific assessment, the European Commission decides whether to approve or reject the novel food application. If approved, the novel food is added to the EU's list of authorised novel foods, and specific conditions of use may be established.
Market Authorisation: Once authorised, novel food ingredients can be placed on the market within the EU, subject to compliance with labelling requirements and any specified conditions of use.
In the US, novel food ingredients are regulated by the Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (FD&C Act). The FDA defines novel foods as those that have not been "generally recognised as safe" (GRAS) by qualified experts for their intended use in food.
The regulatory framework for novel food ingredients in the US involves:
GRAS Notification: The manufacturer or developer of a novel food ingredient may submit a GRAS notification to the FDA, providing scientific evidence to support the safety of the ingredient for its intended use.
FDA Review: The FDA reviews the GRAS notification, evaluating the scientific data and information provided to determine whether the use of the novel food ingredient is safe under the intended conditions of use.
GRAS Determination: If the FDA concludes that the scientific evidence supports the safety of the novel food ingredient, it issues a "no objection" letter, affirming that the ingredient is GRAS for its intended use.
Market Entry: Once a novel food ingredient receives a GRAS determination from the FDA, it can be marketed and used in food products without the need for additional regulatory approval.
Food Safety and Standards Authority of India
FSSAI is the apex regulatory body responsible for ensuring the safety and quality of food products in India. It was established under the Food Safety and Standards Act, 2006. FSSAI lays down the standards for various food products, including novel food ingredients.
Novel food ingredients are those that have not been traditionally used in food products and have no history of safe use. These could include new substances, food additives, or ingredients obtained through new technologies or processes.
Approval Process: FSSAI typically requires novel food ingredients to undergo a safety assessment and approval process before they can be used in food products. This process may involve submission of scientific data and evidence demonstrating the safety of the novel ingredient for human consumption. The approval process ensures that the novel food ingredient does not pose any health risks to consumers.
Safety Assessment: The safety assessment of novel food ingredients may include studies on toxicity, allergenicity, nutritional composition, and any potential adverse effects on human health. This assessment is crucial to determine whether the novel ingredient meets the safety standards set by FSSAI.
Labelling Requirements: Once a novel food ingredient is approved for use, food manufacturers are required to label their products accurately, including information about the presence of the novel ingredient and its concentration. This allows consumers to make informed choices about the food products they purchase.
Monitoring and Enforcement: FSSAI conducts regular inspections and monitors the food market to ensure compliance with safety standards and regulations. Non-compliance can result in penalties or other enforcement actions.
International Standards and Collaboration: FSSAI may also reference international standards and guidelines, such as those established by the Codex Alimentarius Commission, to inform its regulatory framework for novel food ingredients. Collaboration with international regulatory bodies and organisations helps ensure alignment with global best practices.
(Gaikwad is research scholar, College of Food Technology, VNMKV, Parbhani; Dr Kshirsagar is professor and head, Department of Food Engineering, College of Food Technology, VNMKV, Parbhani; Mane is assistant professor, MIT College of Food Technology, Chhatrapati Sambhajinagar. They can be reached at ganeshpg107@gmail.com)
|
|
|
|
|
|
|
|
|
|